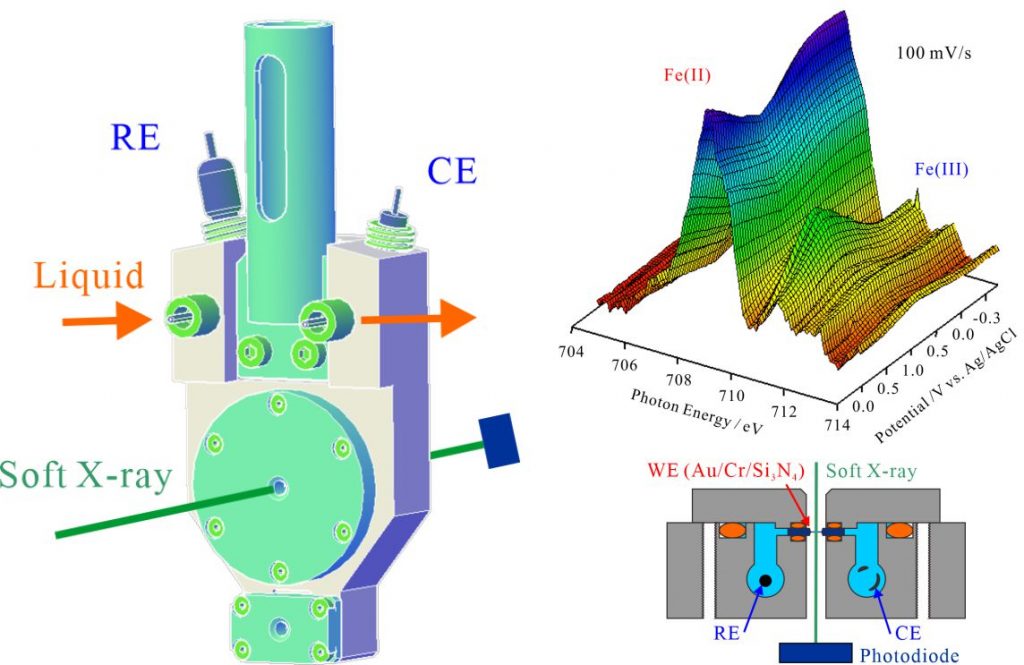
For understanding the mechanisms of electrochemical reactions in solutions, it is necessary to investigate the electronic structures of electrolytes and electric double layers at different potentials. As shown in Fig. 1, the electrochemical cell including working, counter, and reference electrodes were developed for the operando XAS measurements of electrochemical reactions [1, 2]. The working electrode (WE) is the Au deposited Si3N4 membrane, which consists of Au (20 nm) / Cr (5 nm) / Si3N4 (100 nm) layers. The counter electrode (CE) is the Pt wire. The reference electrode (RE) is the Ag/AgCl immersed in saturated KCl or 3M NaCl solutions. Operando XAS measurements were performed by detecting soft X-rays, which transmit Si3N4 membranes that consist of the WE electrode.
The electronic structures of electrolytes can be measured using a thick liquid layer. When the thickness of the liquid layer becomes thinner, the electronic structures of solid-liquid interface on the electrode surface including the electric double layers can be obtained by the operando XAS measurements. The Fe L-edge XAS spectra of aqueous iron sulfate solutions were successfully measured at different potentials with a scan rate of 100 mV/s. The mechanisms of the Fe redox reactions with the change in valence of Fe ions were discussed by correlating the results of cyclic voltammetry with the same scan rates. The present operando XAS measurement opens the possibility of revealing the mechanism of several electrochemical reactions for future applications in energy conversion and storage.