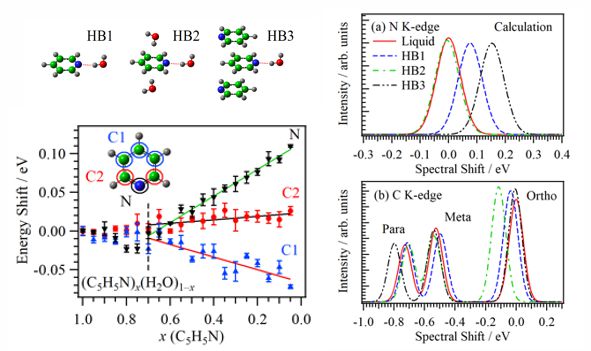
Molecular interactions of pyridine in aqueous solutions (C5H5N)x(H2O)1−x at different molar fractions were studied by XAS at the C, N, and O K-edges. In the pyridine-rich region (x > 0.7), the π* peak energies are not different from neat pyridine (x = 1.0), indicating that antiparallel displaced structures of pyridine molecules are still dominant. In the water-rich region (0.7 > x), the N peaks show higher energy shifts, and the C peaks of the meta and para sites show lower energy shifts by increasing the molar fraction of water. The hydrogen bond (HB) network of bulk water is dominant in this region, but quantum chemical inner-shell calculations indicate that small pyridine clusters still exist in the HB network of water even in dilute solutions.