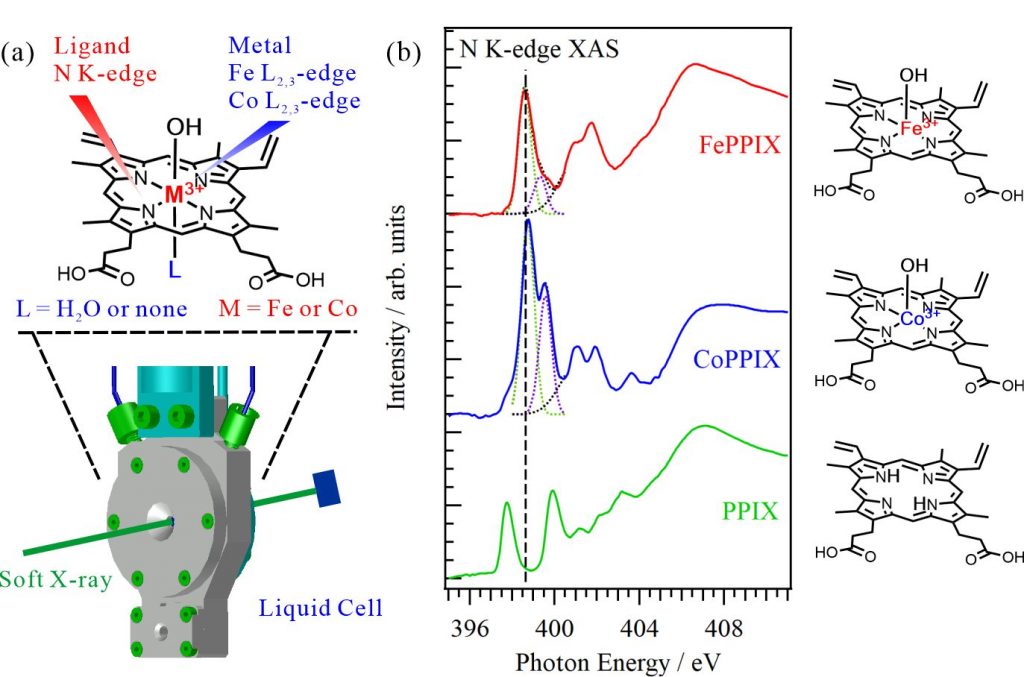
Porphyrin is known as a “pigment of life” because porphyrin and its metal complexes are involved in various biochemical processes. Many metal porphyrin complexes function not in crystal phases but in solutions or protein scaffolds, where their structures can be dynamically changed. In this study, the electronic and coordination structures of metal porphyrin complexes in aqueous solutions were investigated using XAS of the central metal ions at the metal L2,3-edges and ligands at the N K-edges, respectively, using a liquid-cell apparatus shown in Fig. 1(a). Figure 1(b) shows the N K-edge XAS spectra of 50 mM iron protoporphyrin IX (FePPIX), cobalt protoporphyrin IX (CoPPIX), and protoporphyrin IX (PPIX) in 0.5 M NaOH aqueous solutions. The PPIX spectrum presents two C=N π* peaks contributed by two different types of C=N groups, one connected to H atoms, the other not connected to H atoms. The XAS spectra of FePPIX and CoPPIX show two C=N π* peaks derived from delocalization of the metal 3d orbitals to the N 2p orbitals. The CoPPIX spectrum exhibits a higher first peak and a larger energy difference between its multiple peaks than the FePPIX spectrum. The energy differences of the C=N π* peaks between FePPIX and CoPPIX reflect the different electronic configurations and spin multiplicities of the metal porphyrin complexes.
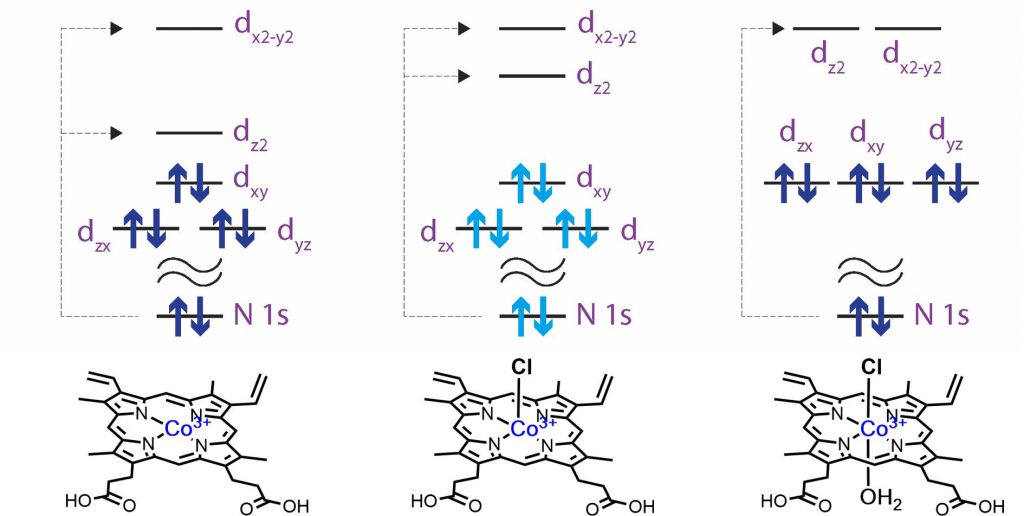
It is known that FePPIX in aqueous solution maintains a five-coordinate geometry, but the coordination geometry of CoPPIX in aqueous solution has not been identified yet. As shown in Fig. 2, the N K-edge inner-shell spectra of CoPPIX with different coordination structures were calculated. The energy difference between dx2-y2 and dz2 orbitals in the planar CoPPIX without counter anion is larger than that in the fire-coordinate CoPPIX. The dx2-y2 and dz2 orbitals are degenerated in the octahedral CoPPIX, resulting in a single C=N π* peaks. Compared to the experiments, it is found that CoPPIX does not form an octahedral structure and maintains its five-coordination geometry in aqueous solutions. In summary, the N K-edge XAS of ligands can directly compare the electronic structures and coordination structures of metal porphyrin complexes in solutions or protein scaffolds.