Time-resolved XAS is an effective method to investigate photoexcitation processes of metal complexes in solutions. As shown in Fig. 1, the time-resolved XAS measurements were performed with the combination of soft X-ray pulses and laser pulses. The soft X-ray pulses with the width of 45 ps were emitted from the soft X-ray beamline BL-13A at KEK-PF during the hybrid mode. The laser pulses (515 nm, 290 fs) were obtained from the second harmonic generation of a Yb:KGW crystal laser. The laser pulses were almost coaxially introduced to the liquid cell with soft X-ray pulses using a center-holed aluminum mirror. The liquid cell is in an atmospheric helium condition, which separates from the soft X-ray beamline and the detector chamber under an ultrahigh vacuum condition using the SiC membrane. The XAS spectra were measured by detecting transmitted soft X-rays using an avalanche photodiode. The Al filter was used for protecting the detector from the laser pulses.
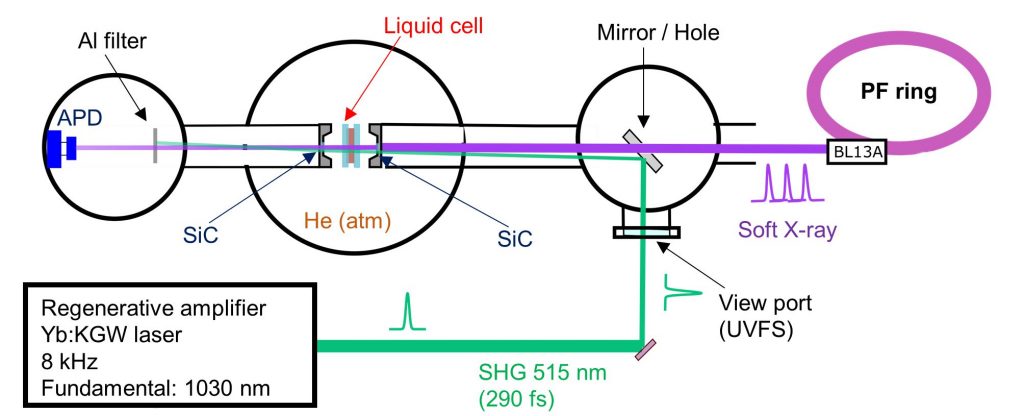
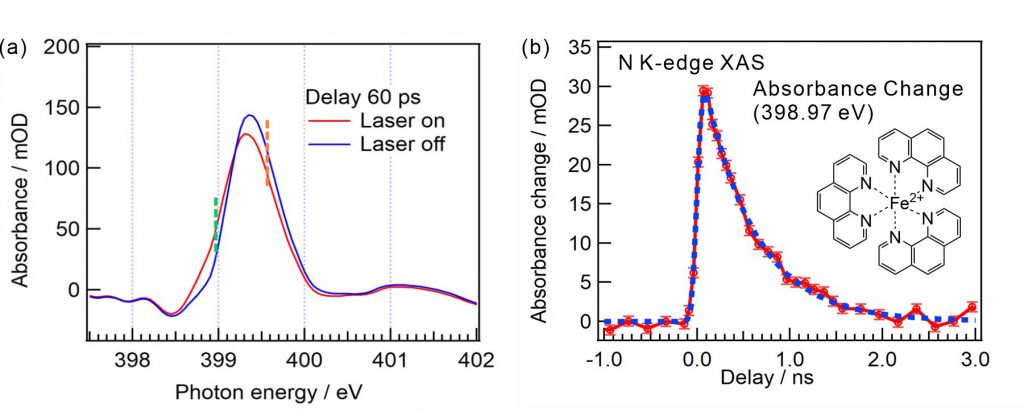
Figure 2(a) shows the N K-edge XAS spectra of [Fe(phen)3]2+ aqueous solutions in the ground and photoexcited states with the 60 ps delay time of soft X-ray pulses from the laser pulses. The C=N π* peak of the ligand in the low spin state is shifted to the lower photon energy owing to the transition to the high spin state by the photoexcitation. Figure 2(b) shows the temporal evolution of the peak intensity difference at 398.97 eV, where the delay times of the soft X-ray pulses were scanned relative to the laser pulses. The obtained time constant of the relaxation process of [Fe(phen)3]2+ complexes from the high spin state to the ground state is 550 ps at the N K-edge, which is close to that obtained by the Fe K-edge (690 ps).