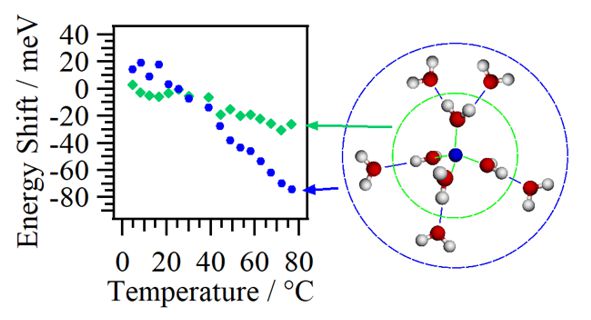
Interaction between water molecules and alkali metal ions in aqueous salt solutions has been studied by the O K-edge XAS. The pre-edge peaks of the hydration water molecules in aqueous salt solutions show higher energy shifts depending on cations but not on anions. The energy shifts of the pre-edge peaks in liquid water at different temperatures represent the structural changes of the hydrogen bond network between water molecules. The pre-edge peaks arising from water molecules in the first hydration shell of lithium cations in aqueous LiCl solutions is not evidently dependent on the temperature, indicating that the hydration water molecules are more strongly bound with lithium cations than other water molecules.