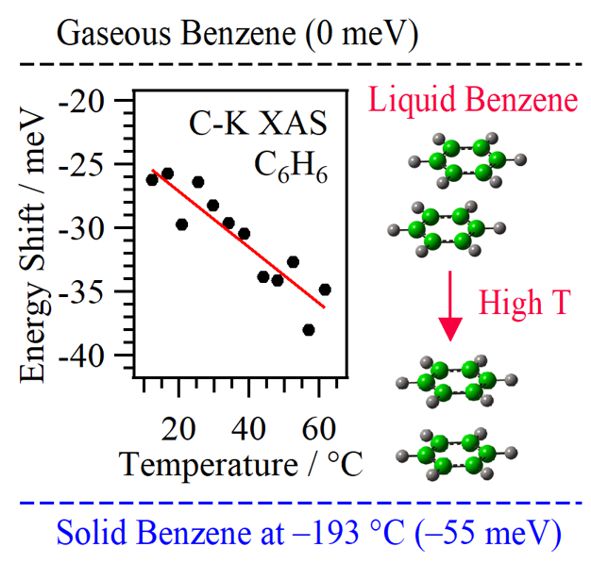
Benzene is the simplest aromatic molecule with π−π interactions. This study addresses fundamental questions regarding whether ordered structures of benzene are formed in the liquid state using C K-edge XAS. The π* peak in liquid benzene unexpectedly shows an opposite temperature behavior, approaching the solid peak apart from the gaseous benzene with increasing temperature. This is rationalized by inner-shell calculations providing insights that structural changes from parallel displaced structures to sandwich (parallel nondisplaced) structures cause the unexpected temperature-dependent spectral shift of the π* peak. These consistent results confirmed that there are temperature-dependent changes of ordered structures of benzene in the liquid state that may affect the mechanisms of chemical and biological phenomena.