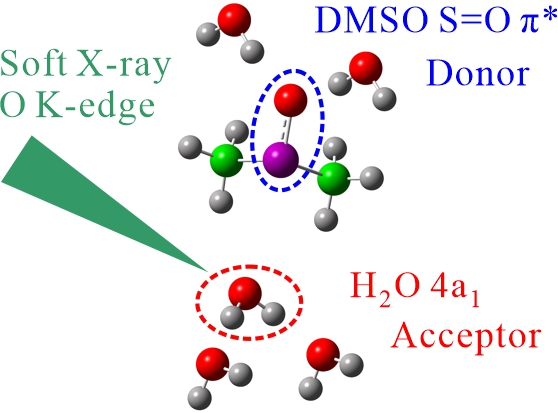
Hydrogen bond (HB) network in aqueous dimethyl sulfoxide (DMSO) solutions at different concentrations has been observed by O K-edge XAS with a site selective analysis that separates donor and acceptor sites of H2O, where the S=O π* peak in DMSO reflects the donor site of H2O and the 4a1 peak in H2O reflects the acceptor site of H2O, respectively. The molecular dynamics simulations and inner-shell calculations revealed that the HB network in aqueous DMSO solutions is influenced with not only the HB interaction of the S=O group with the donor site of H2O but also the dipole interaction of the S atom with the acceptor site of H2O, which breaks the HB network between H2O. Four concentration regions were found in the HB network of aqueous DMSO solutions, which would be related to the anomalies of physical properties and solvent effects in chemical and biological reactions.