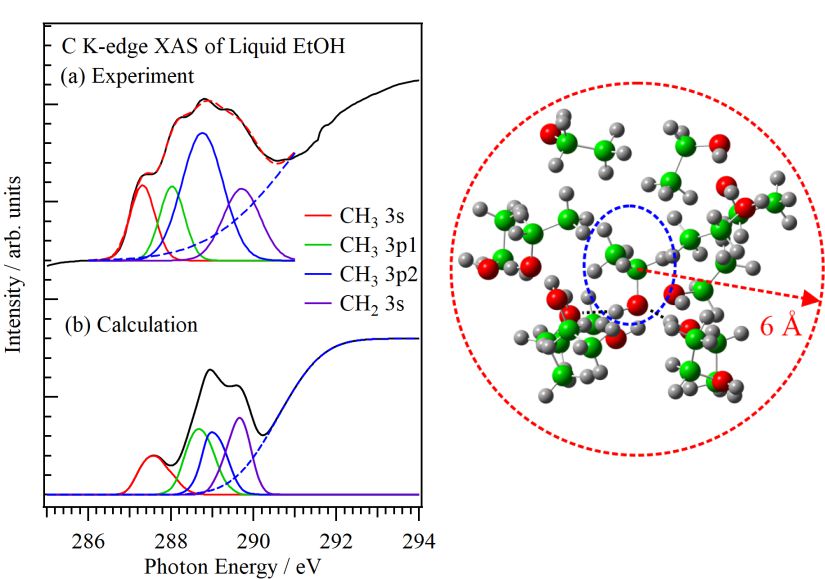
The XAS spectra of liquid samples include information of structural deviations in the liquid phase. For reproducing C K-edge XAS spectra of liquid alcohols such as ethanol and methanol, the inner-shell calculations were performed with the snapshots of the liquid structures obtained by molecular dynamics simulations [1]. Figure 1(a) shows the C K-edge XAS spectra of ethanol in gas and liquid phases obtained by the experiments. Figure 1(b) shows the calculated C K-edge inner-shell spectra of ethanol in gas and liquid phases. The C K-edge inner-shell spectrum of liquid ethanol obtained by the summation of one thousand calculated spectra including neighbor molecules within the CH2‒CH2 distance of 6 Å. The calculated C K-edge inner-shell spectra of liquid alcohols well reproduced the spectral shapes of the experimentally obtained XAS spectra and the spectral changes from gas to liquid phases.
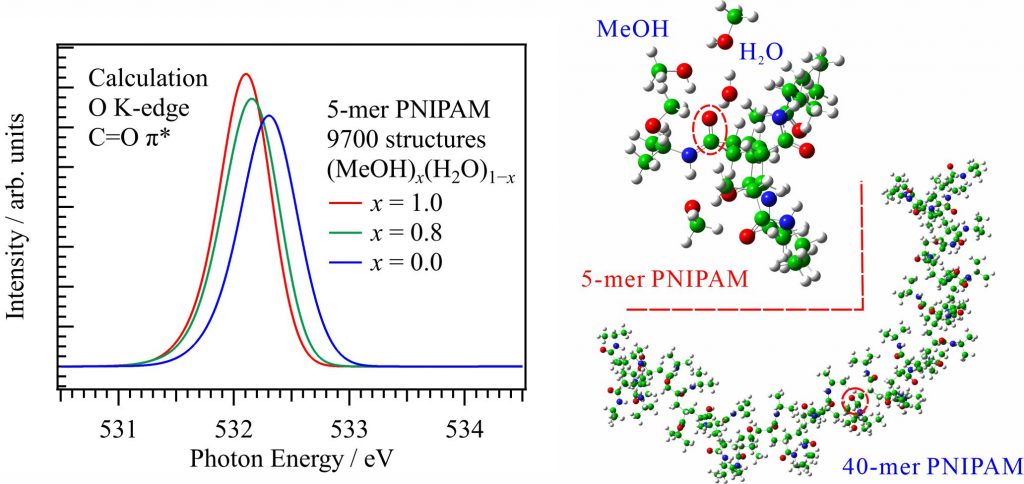
It is important to apply the inner-shell calculations of liquids to large molecular systems, such as polymers and soft matter in solution. For obtaining the O K-edge inner-shell spectra of poly(N-isopropylacrylamide) (PNIPAM) in solutions, the 5-mer PNIPAM chains with terminated H atoms, including the second coordination shells of the solvent methanol and water molecules, were extracted from the 40-mer PNIPAM chains in solutions, whose snapshots were obtained by the molecular dynamics simulations [2]. The inner-shell spectra of PNIPAM in aqueous methanol solutions shown in Fig. 2 were obtained by averaging those of 9700 extracted polymer structures. This calculation method can be precisely evaluated the energy shifts of the C=O π* peaks of PNIPAM caused by the structural changes of the polymer chains, the substitutions of the hydrogen bonds of the C=O groups in PNIPAM from methanol to water molecules, and the increases of the coordination numbers of solvent molecules with the C=O groups, which were observed in the O K-edge XAS experiments.